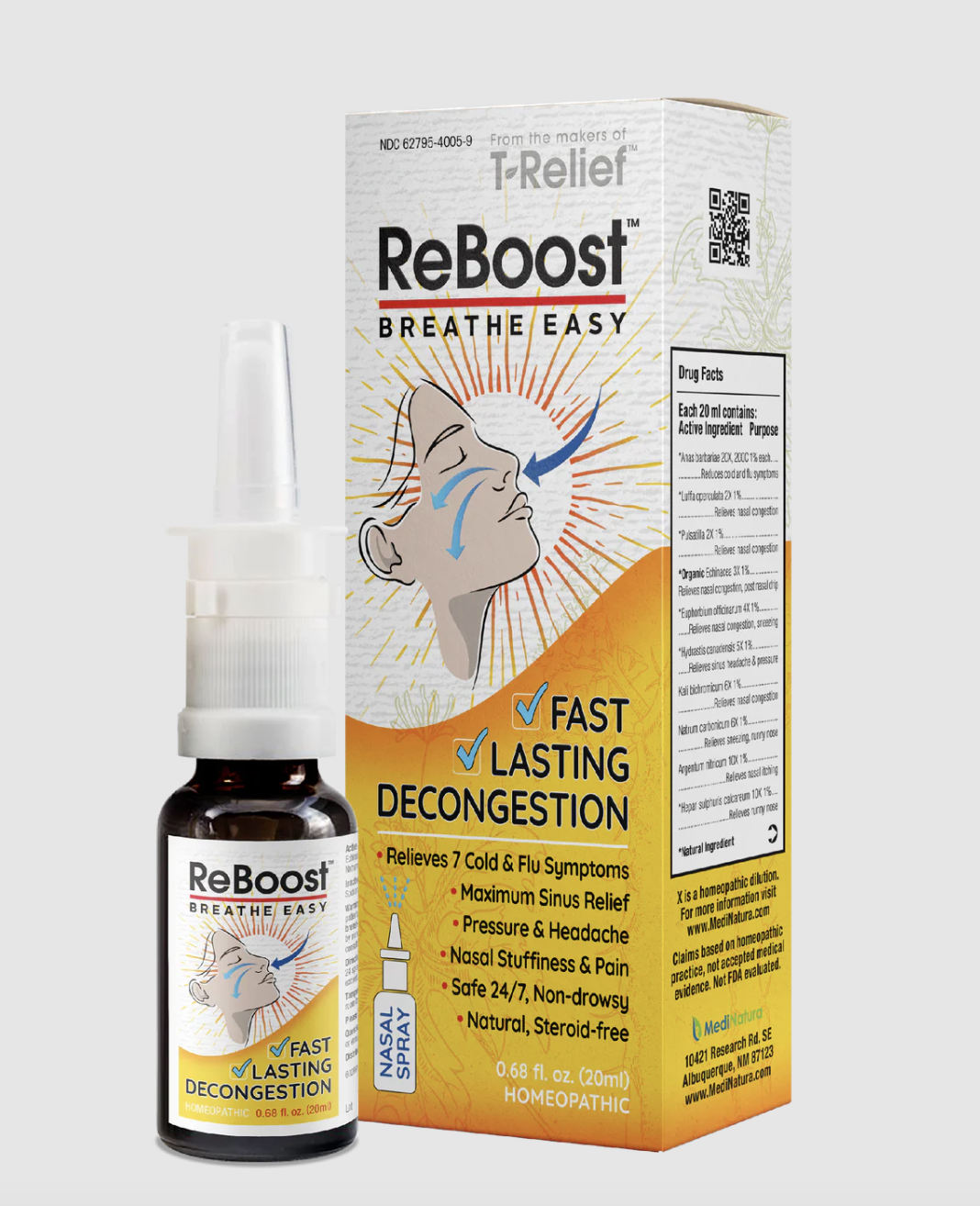
ReBoost Nasal Spray is facing intense scrutiny after health officials confirmed the presence of mold and bacteria in certain batches. The discovery has led to a nationwide recall and raised serious concerns about consumer safety. Authorities have urged users to stop using the product immediately, citing the risk of infections that could become severe in some individuals.
This situation has drawn attention to how contamination in everyday health products can pose unexpected risks.
What Led to the Safety Concerns
The recall was announced after testing found yeast, mold, and microbial contamination in ReBoost Nasal Spray. One bacterium identified during testing was Achromobacter, detected at levels exceeding acceptable safety limits. Health officials stated that exposure to these microorganisms could lead to adverse health effects.
The concern is particularly high for people with weakened immune systems, including older adults, individuals with chronic illnesses, and those undergoing certain medical treatments. Because nasal sprays are applied directly into the nasal passages, contamination can allow harmful microbes to enter the body more easily.
Health Risks and Symptoms to Watch

Using a contaminated nasal spray may lead to a range of symptoms. Health authorities have warned that users should be alert for fever, sinus swelling, headaches, facial pain or pressure, and facial numbness. These symptoms may appear soon after use or develop over time.
Anyone experiencing these warning signs after using ReBoost Nasal Spray should seek medical advice promptly. You may also find it helpful to review our internal article on common infection symptoms and early warning signs to better understand when to act.
How to Identify the Recalled Product
Not every ReBoost Nasal Spray is affected by the recall. Consumers should carefully check the product details listed on the packaging.
| Product Detail | Information |
|---|---|
| Product Name | ReBoost Nasal Spray |
| Lot Number | 224268 |
| Expiration Date | December 2027 |
| Bottle Size | 20 mL |
| Packaging | White and yellow carton |
If your product matches these details, discontinue use immediately.
What Consumers Should Do Now
Customers who purchased ReBoost Nasal Spray directly from the manufacturer are eligible to request a refund through the company’s recall process. Those who bought the product from retail stores or online marketplaces should return it to the place of purchase.
As of the latest update, no confirmed serious adverse events have been reported. Still, health officials emphasize that early action is key to preventing potential complications. Our internal guide on safe nasal spray use offers additional tips for avoiding similar risks in the future.
Why This Recall Is Important
ReBoost Nasal Spray is a homeopathic product made with natural ingredients and marketed to relieve nasal congestion. While many consumers view natural remedies as safer options, this recall highlights that contamination can occur in any product if proper quality controls fail.
The incident serves as a reminder to stay informed about recalls and safety alerts. Checking product labels, following official guidance, and monitoring your health can make a significant difference.
By remaining aware and taking prompt action, consumers can better protect themselves and their families while regulators continue to review product safety standards.